Fraunhofer IFAM spin-off receives CE Mark for medical device for effective removal of kidney stones

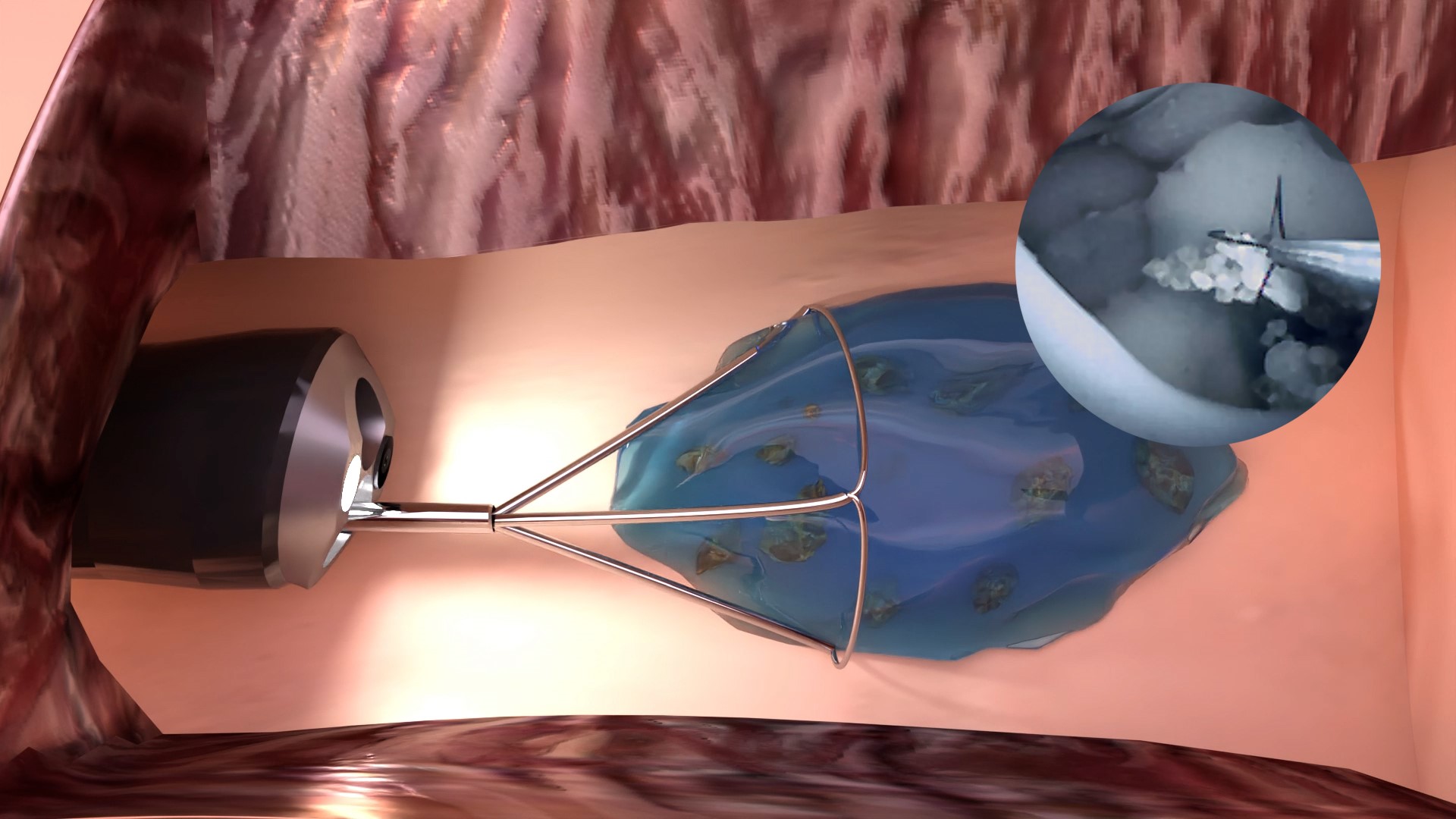

In December 2017, Purenum GmbH started its business as a spin-off of Fraunhofer IFAM. Its mission is to develop biomimetic adhesives for use in medical technology. Bremen based Purenum GmbH has now succeeded in certifying a biocompatible hydrogel for the removal of kidney stone residues for endoscopic therapy. This milestone paves the way for clinical application on patients.
In Germany alone, there are over one million annual cases of treatment for kidney stone disease. Endoscopic treatments are performed on many thousands of patients to remove kidney stones. Smaller stone debris, however, cannot yet be reliably removed because it is too small to grasp and thus often remains in the kidney.
Kidney stone fragments intelligently removed
"With the now CE marked medical hydrogel, which will be distributed under the product name "mediNiK®", it is possible to remove even the smallest kidney stone fragments without additional complicated procedural steps during endoscopy" explains Manfred Peschka, Managing Director of Purenum GmbH. "After removing the large kidney stones, the remaining residual fragments are encapsulated with the "mediNiK®" hydrogel. The resulting conglomerate of hydrogel and stone fragments is large enough to be easily removed with standard instruments", Manfred Peschka describes the novel procedure. "The successful certification and the resulting declaration of
conformity guarantees the safety and performance of the product. It is the most important regulatory hurdle for the use of a medical device. To provide all German patients with access to this effective medical method, our next step will be to clarify the coverage of the treatment by health insurers. We are planning another six months for this process so that “mediNiK®” can be used for all patients in Germany at the beginning of 2022", Manfred Peschka is convinced.
From research to application
"Adhesive development for use in medical technology is a research focus at Fraunhofer IFAM. Before a development can go into use as a medical product, many years of preliminary research have been carried out and numerous hurdles must be overcome before certification can be obtained. We are therefore even more pleased, together with the Purenum GmbH research team, about this important success", says Prof. Bernd Mayer, Director of Fraunhofer IFAM. "We experienced the beginnings of the research project at the institute and saw the great commitment with which the former employees of Fraunhofer IFAM drove the work forward and applied their expertise in a goal-oriented manner. With this energy, the team has successfully made the long journey from the product idea to a professional medtech company. We are convinced that "mediNiK®" will decisively improve the removal of kidney stone fragments", says Bernd Mayer.
About Purenum GmbH
Purenum GmbH develops and researches new hydrogels and adhesive systems for applications in the field of medicine; these are primarily systems that are used on or in the patient as medical devices. The services also include technical adhesives that are used, for example, in the manufacture of medical devices (or other application scenarios).
Funding
Purenum GmbH is financed by various private and public investors.


Press release and pictures for download
Last modified:
 Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM
Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM