How to avoid galvanic corrosion?
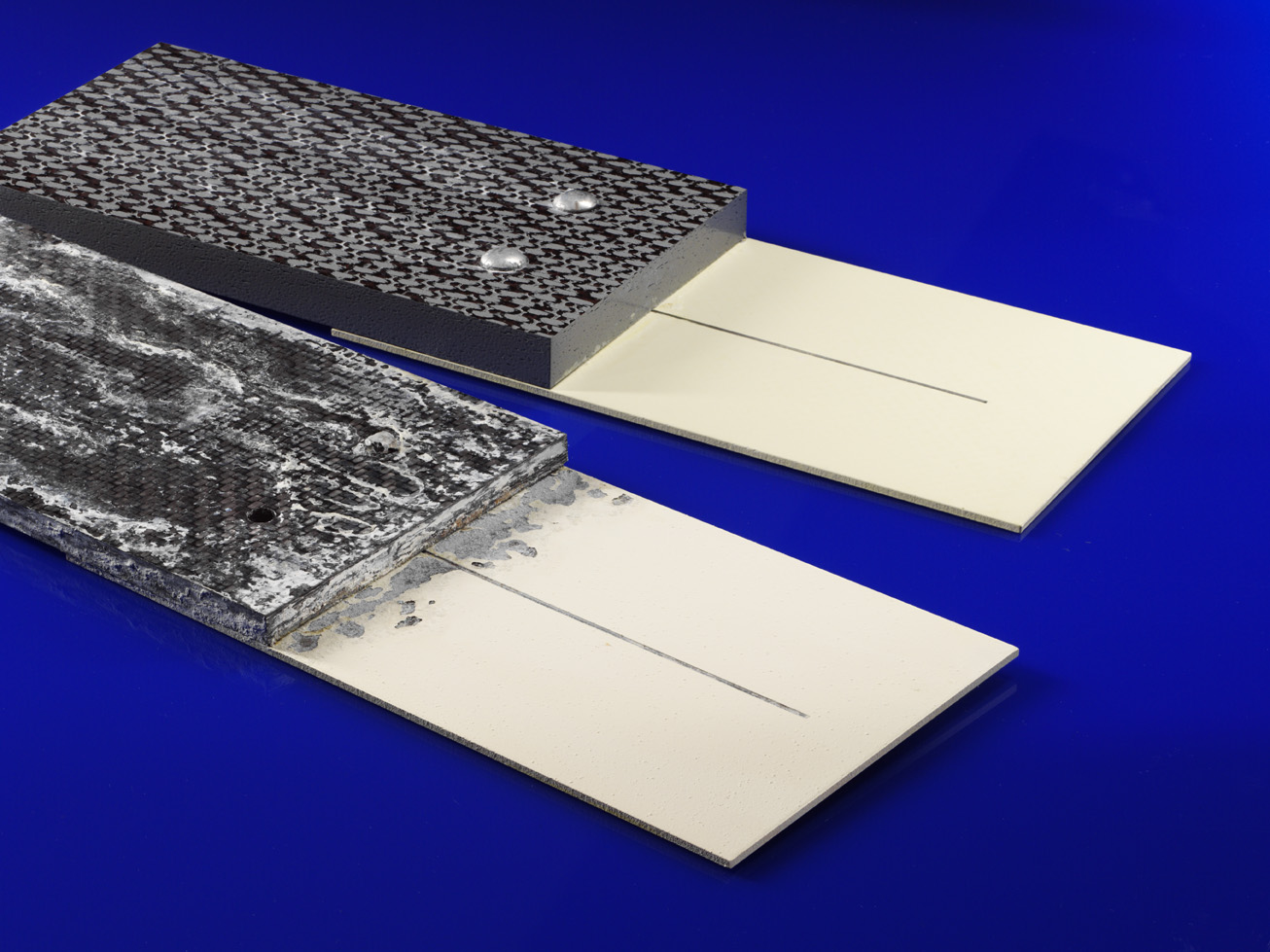
Galvanic corrosion, also called bimetallic corrosion or dissimilar metal corrosion is not a new phenomenon: Even in ancient times, it was found that copper nails used to fasten lead sheets to ship hulls corroded more quickly. Although this phenomenon has been known for centuries, this form of corrosion continues to cause damage and accidents. The experts at Fraunhofer IFAM can support in risk assessment and the selection of protection methods.
Reaction between different metals
Galvanic corrosion is caused by the electrochemical reaction of two different metallic materials - e.g. steel and aluminum - or other electrically conductive materials, e.g. carbon fibers, which form the electrodes. The less noble metal forms the anode and dissolves accelerated, while the more noble metal forms the cathode.
Galvanic corrosion can occur when two different conductive materials are in electrical and galvanic contact. The latter requires the simultaneous immersion of both materials in the same liquid (electrolyte). In addition, an unfavourable surface area ratio (large cathode, small anode) can lead to a strongly accelerated dissolution, because the cathodic partial reaction is intensified by a larger cathode surface area and the corresponding anodic partial reaction is concentrated on a small surface area.
If carbon fiber reinforced plastics (CFRP) are involved, the carbon fibers always form the cathode. Here free fibers e.g. at cutting edges in contact with the electrolyte pose the danger.
How to avoid galvanic corrosion
If critical material pairings cannot be avoided, protection measures based on the following principles can be considered:
- Avoid electrical contact
- Avoid galvanic contact
- Increase system resistance
- Compatible material pairings
Depending on the application, however, protection measures has to be evaluated in terms of effectiveness and applicability. Unfortunately, not every measure that completely minimizes the risk of corrosion can always be technically implemented.
Thanks to their extensive know-how in the field of corrosion, our scientists are able to provide you with the best possible support in assessment of galvanic corrosion risk. Not only the selection and recommendation of protective measures is part of our portfolio, but also the validation of corresponding protective measures by means of corrosion testing.
 Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM
Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM