Determination of reaction enthalpies of moisture-sensitive materials under inert gas atmosphere
The enthalpy measurement of mixing reactions during the synthesis of air-sensitive materials must be carried out under inert conditions and thus places special demands on the measurement setup. One example of moisture-sensitive materials are sulfide solid-state electrolytes, which may be used in future solid-state batteries (ASSBs). To make these materials economically attractive, Fraunhofer IFAM is researching the scale-up of the synthesis process. For the upscaling of the syntheses, reaction enthalpies are required, which must be measured in an inert gas atmosphere.
Production of new battery materials
Considering the ever-increasing demand for energy and the need to store renewable forms of energy, the further development of electrical energy storage systems is essential. Solid-state batteries are considered a new generation of batteries. Here, the liquid electrolyte of conventional batteries is replaced with a solid electrolyte, which has many advantages.
In this context, sulfidic materials as solid-state electrolytes offer advantages in terms of ductility, and, thus, are easy to process as well as good intrinsic ionic conductivity. In contrast, sulfidic materials are challenging to synthesize and process due to moisture sensitivity.
While many sulfidic materials have been studied extensively at the laboratory scale for their suitability for application and processability, the production of larger quantities of materials has been of minor interest. To scale up a synthesis, many aspects must be kept in mind. One component is the energetic consideration of the synthesis. The synthesis steps must be studied in terms of their enthalpies to estimate heat generation and heat requirements. Excessive heat generation can lead to serious safety problems such as bursting of reaction vessels or leakage of toxic gases, or simply degeneration of the reactants. However, if too much heat is removed or not enough is added, the reaction slows down or even stops completely.
Reaction enthalpies of syntheses of sulfidic solid-state electrolytes
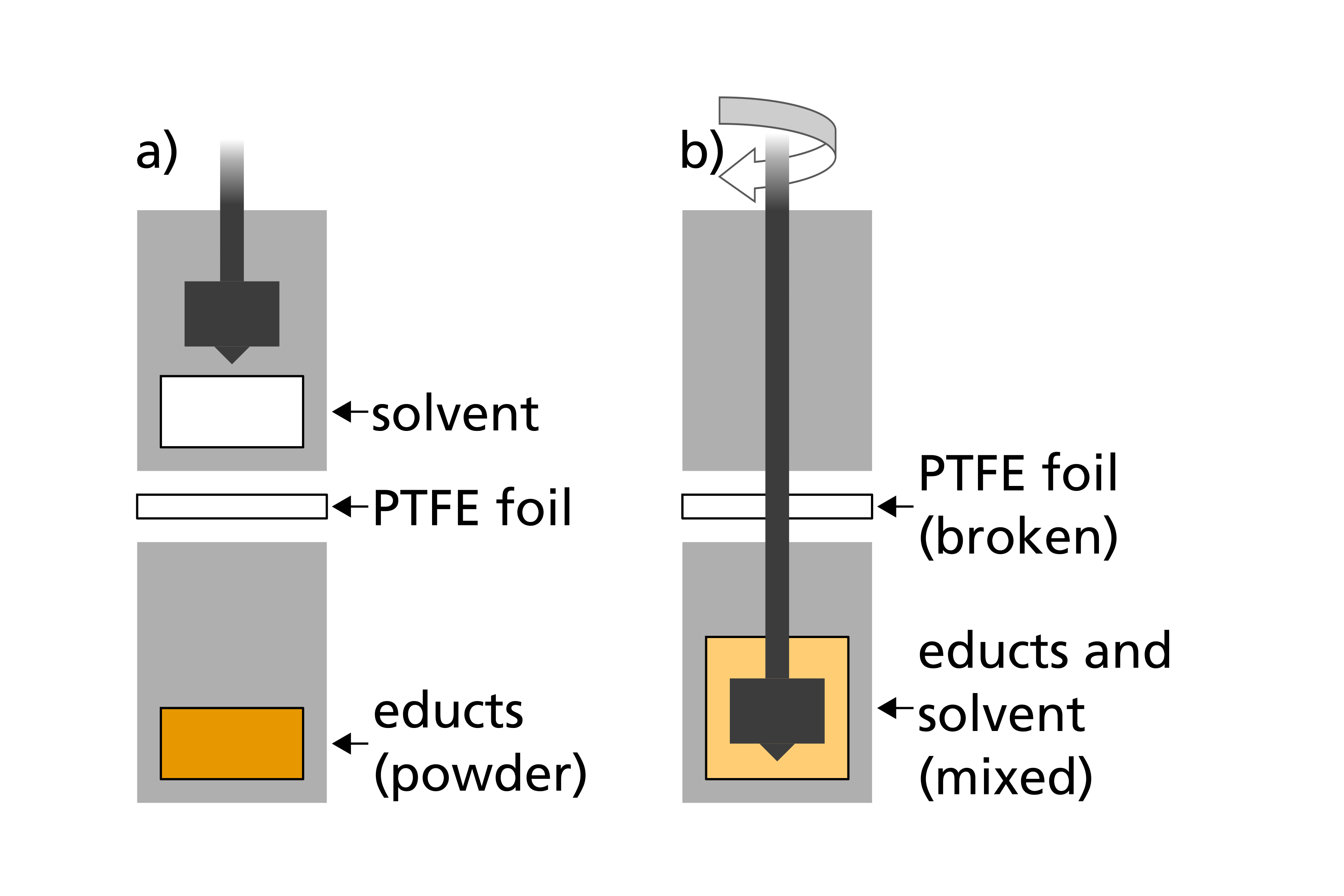
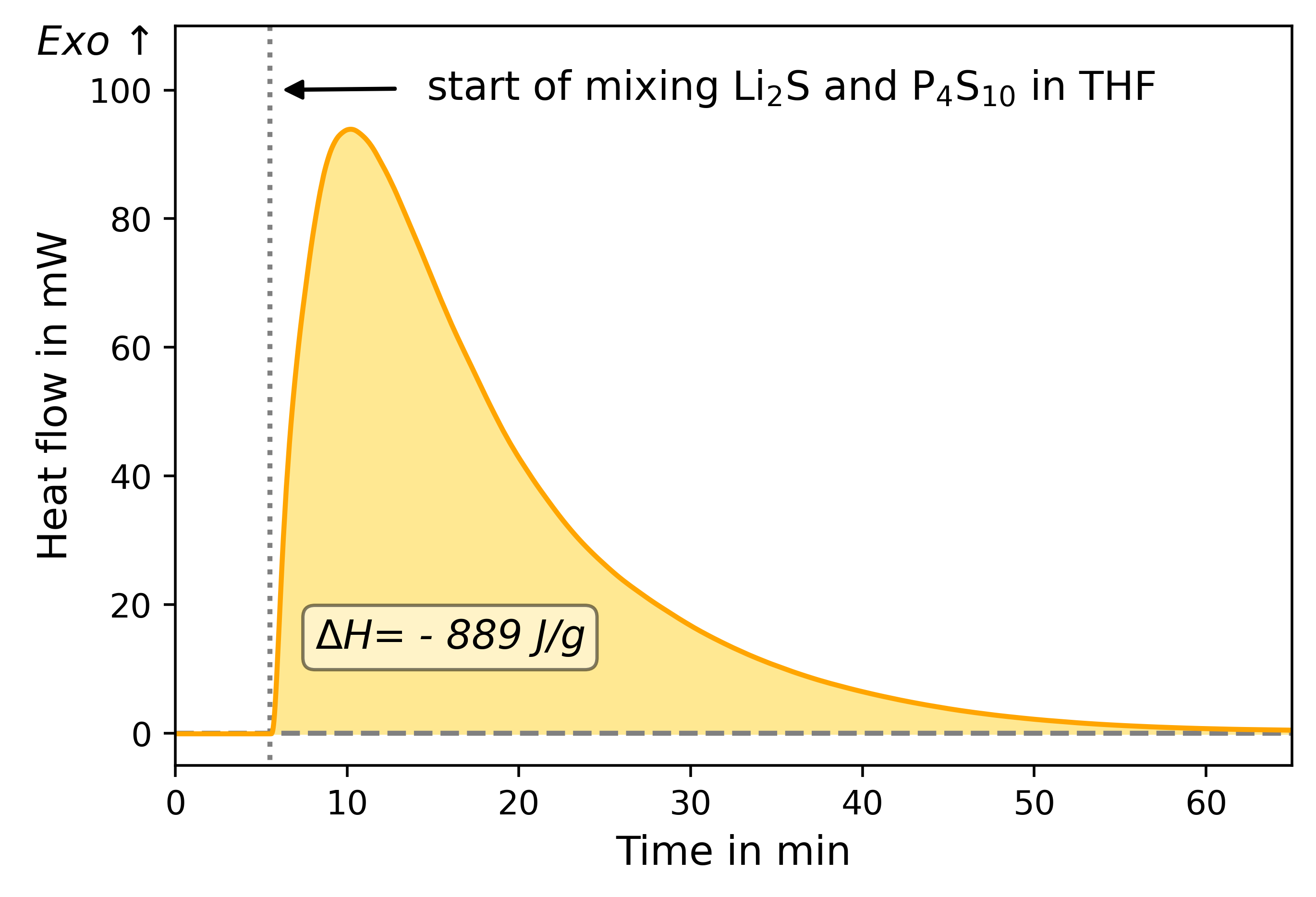
Since both the starting materials (reactants) and the products of the synthesis of sulfidic solid-state electrolytes react with moisture in the ambient air, an inert gas atmosphere must be ensured during the enthalpy measurement. This is particularly challenging in the case of the wet-chemical synthesis, since the reaction starts as soon as the solid and liquid reactants come into contact with each other. A sample preparation must therefore be carried out in such a way that the reactants cannot yet react with each other, but are prepared under an inert gas atmosphere and are only mixed once the measurement is started.
 Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM
Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM