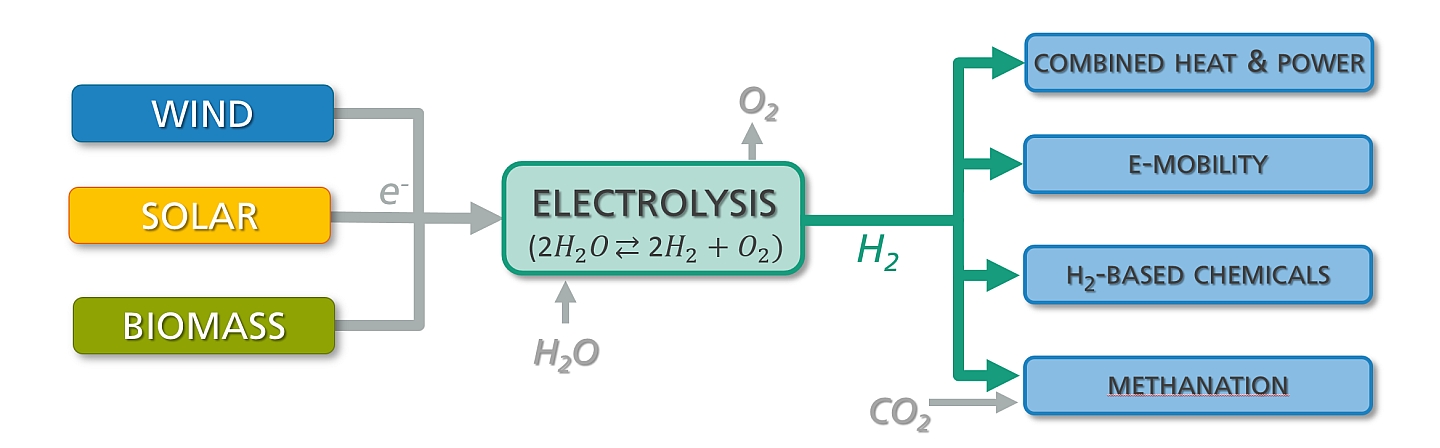
In view of the shortage of fossil energy resources and the ongoing climate change caused by man-made carbon dioxide emissions, the energy industry is facing a radical change. In view of “green” energy technologies, hydrogen will play a leading role as secondary energy carrier if a low-cost and climate-neutral hydrogen production from renewable energy sources like wind or solar power can be achieved. The alkaline electrolysis of water has the potential to fulfill both requirements. However, in the last years only marginal efforts have been made to enhance efficiency and power density of the electrolysis process.
Electrolysis
3D Electrodes
Porous materials offer the possibility to enlarge the active surface area of the electrode and to direct the gas-electrolyte flow. Both aspects depend on the pore structure. At Fraunhofer IFAM Dresden we are able to modify open-cellular metal foams or fleeces so that they form nanocrystalline surfaces which are electrocatalytically very active:
- pore size: 5 - 1200 µm
- porosity: 50 - 95 vol.%
Nanocrystalline Electrodes
The electrocatalytical activity of electrodes and thus the efficiency of the electrolysis process are positively influenced by nanocrystalline electrode surfaces. Such a nanocyrstallinity of metal surfaces can be achieved by certain surface treatments or deposition processes. At Fraunhofer IFAM Dresden, nanocrystalline metal alloys (Fe- and Ni-base), which are produced by melting metallurgical routes, are being developed and tested with regard to their applicability as electrode materials.
Electrochemical and Structural Analysis
Fraunhofer IFAM Dresden is equipped with state-of-the-art analysis tools in order to investigate the electrochemical properties, the surface morphology and the structural properties of the electrode materials:
- Electrochemical analysis
- Cyclic voltammetry (CV)
- Electrochemical impedance spectroscopy (EIS)
- Polarization methods
- Scanning electron microscopy (SEM)
- Scanning probe microscopy
- Atomic force microscopy (AFM)
- Scanning tunneling microscopy (STM)
- Electrochemical STM (EC-STM) and scanning electrochemical potential mapping (SECPM) for in situ experiments in liquids
Lab-scale Electrolyzer
The electrode materials are tested under realistic operation conditions (80°C, 30 m% KOH) to demonstrate their applicability. Therefore, the electrodes are integrated into lab-scale electrolyzer cells. Due to the flexible design of the test system different electrode configurations and operation conditions can be realized:
- Single-cell design
- 20 °C ... 80 °C
- atmospheric pressure
- 5 Normliter-H2 per hour
Literature
C. I. Bernäcker, T. Rauscher, T. Büttner, B. Kieback, L. Röntzsch
A Powder Metallurgy Route to Produce Raney-Nickel Electrodes for AlkalineWater Electrolysis
Journal of the Electrochemical Society, 166 (6), 2019, F357-F363
M. Durovic, J. Hnát, C. I. Bernäcker, T. Rauscher, L. Röntzsch, M. Paidar, K. Bouzek
Nanocrystalline Fe60Co20Si10B10 as a cathode catalyst for alkaline water electrolysis: Impact of surface activation
Electrochemica Acta 306, 688-697, 2019
M. Farjad Ali, H. In Lee, C. I. Bernäcker, T. Weißgärber, S. Lee, S.-K. Kim, W.-C. Cho
Zirconia Toughened Alumina-Based Separator Membrane for Advanced Alkaline Water Electrolyzer
polymers, 14 (6), 2022, 1173
F. Foroughi, C. I. Bernäcker, L. Röntzsch, B. G. Pollet
Understanding the Effects of Ultrasound (408 kHz) on the Hydrogen Evolution Reaction (HER) and the Oxygen Evolution Reaction (OER) on Raney-Ni in Alkaline Media
Ultrasonics Sonochemistry, Vol. 84, 2022, 105979
A. Gabler, C. I. Müller, T. Rauscher, T. Gimpel, R. Hahn, M. Köhring, B. Kieback, L. Röntzsch, W. Schade
Ultrashort-pulse laser structured titanium surfaces with sputter-coated platinum catalyst as hydrogen evolution electrodes for alkaline water electrolysis
International Journal of Hydrogen Energy, Vol. 43, Issue 15, 2018, 7216-7226
A. Gabler, C. I. Müller, T. Rauscher, M. Köhring, B. Kieback, L. Röntzsch, W. Schade
Ultrashort pulse laser-structured nickel surfaces as hydrogen evolution electrodes for alkaline water electrolysis
International Journal of Hydrogen Energy, Vol. 42, Issue 16, 2017, 10826-10833
H. A. Miller, K. Bouzek, J. Hnat, S. Loos, C. I. Bernäcker, T. Weißgärber, L. Röntzsch, J. Meier-Haack
Green hydrogen from anion exchange membrane water electrolysis: a review of recent developments in critical materials and operating conditions
Sustainable Energy Fuels, 4, 2020, 2114-2133
C. I. Müller, K. Ehelebe, S. Loos, L. Röntzsch
Elektrochemische Reduktion von CO2 zu eChemicals: C242
gwf Gas+Energie, 159, 2018, 52–56
C. I. Müller, K. Sellschopp, M. Tegel, T. Rauscher, B. Kieback, L. Röntzsch
The activity of nanocrystalline Fe-based alloys as electrode materials for the hydrogen evolution reaction
Journal of Power Sources 304, 2016, 196-206
C. I. Müller, T. Rauscher, A. Schmidt, T. Schubert, T. Weißgärber, B. Kieback, L. Röntzsch
Electrochemical investigations on amorphous Febase alloys for the alkaline water electrolysis
International Journal of Hydrogen Energy, Vol. 39 Issue 17, 2014, 8926–8937
T. Rauscher, C. I. Bernäcker, S. Loos, M. Vogt, B. Kieback, L. Röntzsch
Spark-plasma-sintered porous electrodes for efficient oxygen evolution in alkaline water electrolysis
Electrochimica Acta, Issue 317, 2019, 128-138
T. Rauscher, C.I. Bernäcker, U. Mühle, B. Kieback, L. Röntzsch
The effect of Fe as constituent in Ni-base alloys on the oxygen evolution reaction in alkaline solutions at high current densities
International Journal of Hydrogen Energy, Vol. 44, Issue 13, 2019, 6392-6402
T. Rauscher, C.I. Müller, A. Gabler, T. Gimpel, M. Köhring, B. Kieback, W. Schade, L. Röntzsch
Femtosecond-laser structuring of Ni electrodes for highly active hydrogen evolution
Electrochimica Acta, Vol. 247, 2017, 1130-1139
T. Rauscher, C.I. Müller, A. Schmidt, B. Kieback, L. Röntzsch
Ni-Mo-B alloys as cathode material for alkaline water electrolysis
International Journal of Hydrogen Energy 41, 2016, 2165-2176
 Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM
Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM