Motivation and Business Activities

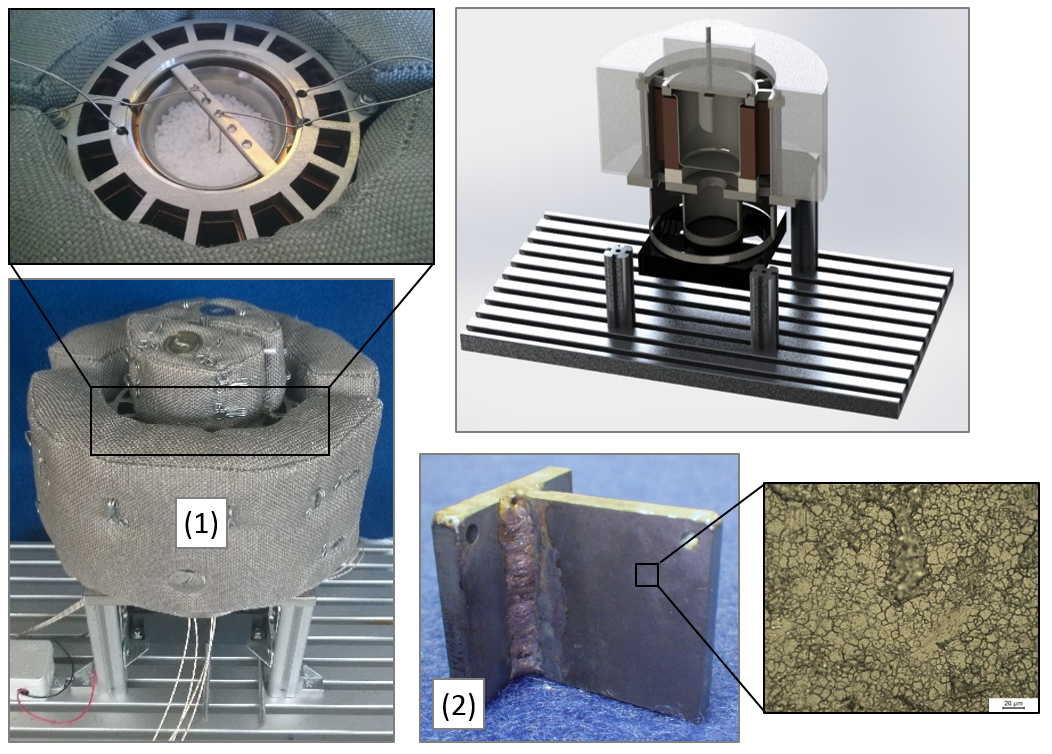
In latent thermal storage, phase change materials (PCM ... phase change materials) are used as storage media, whereby the phase change is solid/liquid and thus melting heat is used to store thermal energy.
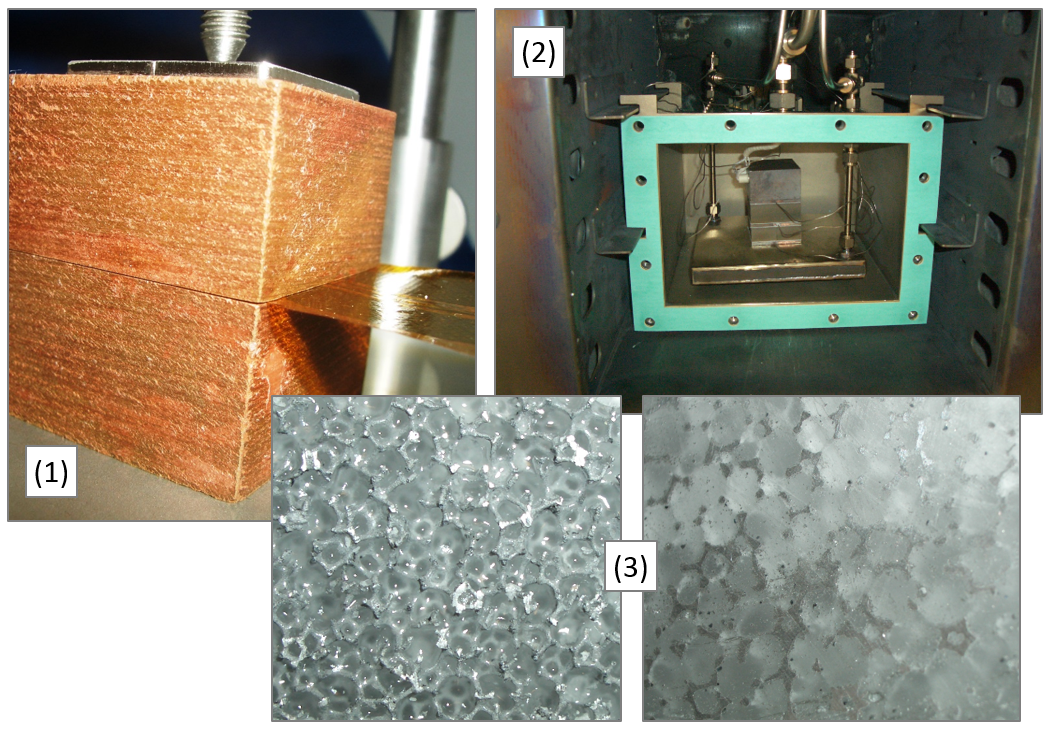
For the design and development of high performance heat storages, it is of particular importance to have access to reliable information regarding the thermophysical material data of PCMs and their interaction with the container or capsule materials. Important parameters are
• melting temperature and the specific heat of fusion,
• specific heat capacity and thermal conductivity,
• density, as well as
• corrosiveness compared to encapsulation materials.
With our available laboratory equipment, we are able to determine many of these quantities - in part also under cyclical thermal load - for typical PCM classes such as
• organic PCM (paraffins, fatty acids, sugar alcohols, etc.) and
• inorganic PCM (salt hydrates, nitrate and nitrite salts)
More detailed information is provided below.
 Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM
Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM